News & Advice

Elementary essentials #1: Nitrogen (N)
In our elementary essentials series, Ravensdown Chief Scientific Officer Dr Ants Roberts delivers the ‘skinny’ on the crucial nutrients that feed our farming systems so we can feed discerning consumers wanting safe, efficiently produced, quality foods.
It has been posited that half the current world’s population would not have been born if it were not for the invention of ammonia fertiliser by German scientists Haber and Bosch in the early 20th century. This is because of the essential requirement of N by plants and animals, and hence food production, to support world population growth. The element nitrogen (N), is the seventh element in the periodic table and is one of the 19 elements essential for life on planet Earth. Despite this, all known life on Earth is referred to as carbon-based lifeforms and it would be impossible for life on Earth to exist without carbon (C) because it is the main component (approximately 45-50% of dry biomass) of most tissues in living things.
Protein synthesis
Carbon is basic to life because it can form stable bonds with many elements, which allows it to form up to 10 million carbon- based molecules in living organisms. Carbon bound to hydrogen (H) and oxygen (O) form the carbohydrates, which are the building blocks of compounds from simple sugars used for energy to structural lignin (eg, wood) in plants. The ability of C to bond with N (together with H and O) forms the building blocks for protein formation.
Proteins are essential in living organisms because they do most of the work in living cells, in terms of structure, function and regulation of tissues. Proteins, and hence N, are essential for making cell membranes, enzymes and nucleic acids (ie, DNA), transporting oxygen, being part of the immune system and messaging between cells.
Food production
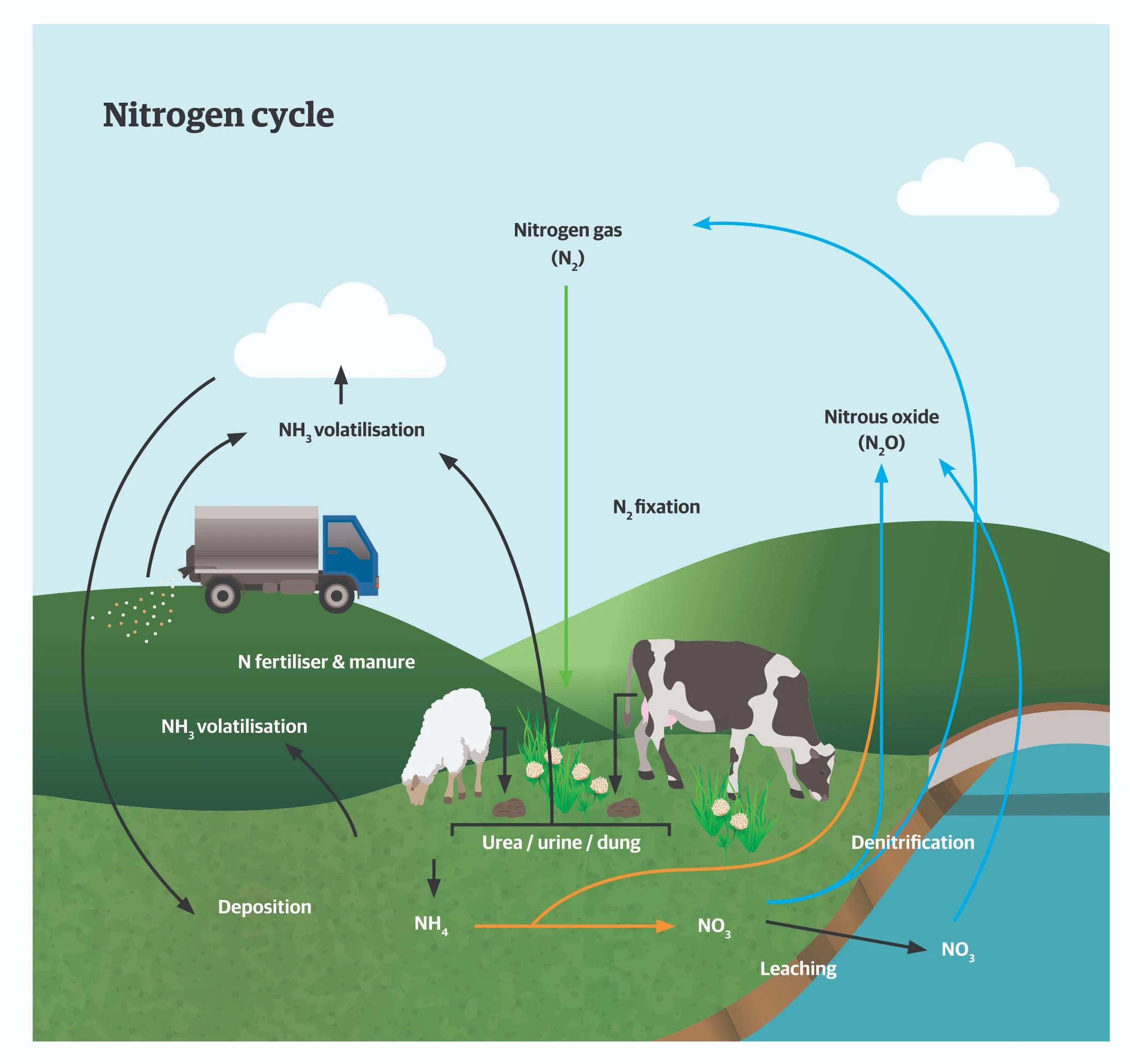
All plants, for both human and animal consumption, extract most of their N requirements from the soil solution. Soil solution N is derived mostly from the organic matter in the soil, which is mineralised (decomposed) by the action of soil micro-organisms, as well as from N fertilisers. Soil organic matter accumulates from death and decay of uneaten leaves and stems, dung and urine, crop residues including roots.
Plants take up soil N as either ammonium or nitrate ions and use these to produce amino acids and proteins. Legumes, eg, clovers, lucerne, beans and peas, can form symbiotic relationships with a group of soil bacteria from the Rhizobium family. Legumes allow rhizobia to infect their root systems to form nodules within which rhizobia extract nitrogen gas from the air to form ammonium ions. The rhizobia exchange some of this ammonium in return for carbohydrate (energy) supplies from the legume host. Trial work has shown that in grazed-pasture situations N fixation by legumes can contribute between 25-150kg
N/ha/year (depending on clover content, climate, topography and slope) to the N cycle. Free-living N fixing microorganisms also live in soils and make a small contribution to the N cycle. Animals and humans get proteins and amino acids from eating plants and then synthesise their own proteins from these.
The N cycle in agricultural systems is leaky and losses of N from soil to the air as ammonia, nitrogen gas or nitrous oxide occur under certain soil conditions and contribute to greenhouse gas emission. Surplus N in soils (from excess fertiliser
N or urine of grazing animals) can contribute to N in drainage water, which affects receiving-water quality. Therefore, we must all try to make the N cycle as efficient as possible so that we can enjoy the benefits of healthy, nutritious food production while minimising any effects on the environment we all live in.
Written by Dr Ants Roberts, Ravensdown Chief Scientific Officer